More Information
Submitted: February 08, 2022 | Approved: March 14, 2022 | Published: March 15, 2022
How to cite this article: Langoya OD, Nampogo AM, Irene A. Spinal cord involvement in tuberculous meningitis: a case report and brief review of literature. J Clin Intensive Care Med. 2022; 7: 001-004.
DOI: 10.29328/journal.jcicm.1001040
Copyright License: © 2022 Ningrum EFS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Tuberculous Meningitis, Spinal Cord, Human Immunodeficiency Virus (HIV)
Spinal cord involvement in tuberculous meningitis: a case report and brief review of literature
Oriba Dan Langoya1,2* , Adrian Mwota Nampogo1 and Andia Irene1
, Adrian Mwota Nampogo1 and Andia Irene1
1Department of Medicine, School of Medicine, Makerere University College of Health Sciences, Kiruddu National Referral Hospital, Kampala, Uganda
2Department of Medicine, St. Mary’s Hospital Lacor, Gulu, Uganda
*Address for Correspondence: Oriba Dan Langoya, MBChB, Department of Medicine, School of Medicine, Makerere University, Upper Mulago Hill Road, P.O. Box 7072, Kampala, Uganda, Email: [email protected]
Introduction: Tuberculosis (TB) continues to pose a significant public health problem worldwide. Tuberculous meningitis (TBM) is the most devastating form of extrapulmonary TB however other forms of central nervous system (CNS) disease include tuberculoma and spinal arachnoiditis. TBM carries high mortality even for a patient who is already receiving treatment. The difficulty in diagnosis often leads to a delay in treatment and subsequent mortality. The emergence of Xpert ultra has improved the rapid detection of MTB and rifampicin resistance in CSF and is the preferred diagnostic tool in TBM.
Case: In this case report we present a 33years patient of concern who presented with progressive lower limb weakness associated with pain and paresthesia for 4 months, admitted via the Orthopedic unit with a diagnosis of spinal mass (meningioma, neurofibroma, or nerve sheath tumor) for which biopsy was done and revealed a chronic inflammatory process, necrotic bone lesions with no granulomas and no malignancy, he was later diagnosed with tuberculous meningitis and promptly started anti-tuberculous therapy with a dramatic recovery and improvement in neurological function.
Conclusion: Tuberculous meningitis conditions have high morbidity and mortality yet diagnosis and start of treatment continue to experience an important delay. Clinicians should keep in mind the limitations of clinical presentation due to pleiotropy and current diagnostics and should employ a combination of diagnostic modalities in addition to a high index of suspicion to prevent morbidity in patients with TBM.
TB is one of the top 10 causes of death and the leading cause of a single infectious agent (above HIV/AIDS). Globally, a total of 1.4 million people died from TB including 208,000 people with HIV in 2019. Whilst ending the TB epidemic by 2030 is among the health targets of the United Nations Sustainable Development Goals (SDGs), HIV and TB continue to form a lethal combination, each speeding the other’s progress [1,2]. TBM is the most severe form of extra-pulmonary TB and adults who live in endemic areas or those who are immunosuppressed due to HIV or other immunosuppressive medications are susceptible to infection [3]. Diagnosis of TBM is difficult for clinicians as it can clinically present similarly to other forms of meningitis. The difficulty in diagnosis often leads to a delay in treatment and subsequent mortality [3,4]. TBM carries a high mortality rate of 30% - 40%, including those receiving treatment and those who survive are left with long-term sequelae leading to lifelong disability. People living with HIV (PLHIV) who develop TBM have a higher mortality rate of > 60% [3,5]. The microbiologic diagnosis of TBM requires the isolation of Mycobacterium tuberculosis (MTB) from the cerebrospinal fluid (CSF) of an infected patient [5]. Unfortunately, many cases of TBM cannot be confirmed based on clinical and imaging findings as the clinical manifestations are nonspecific, while laboratory techniques are largely insensitive or slow [3]. The emergence of Xpert ultra has improved the rapid detection of MTB and rifampicin resistance in CSF samples however a trace-positive Xpert Ultra result cannot provide drug resistance information [6]. In this case report, we emphasize the pleiotropic nature of presentation in TB meningitis with a dramatic recovery and highlight the importance of gene Xpert ultra in establishing the diagnosis. Xpert Ultra adds 2 new MTB target genes (IS1081 and IS6110) and provides a larger capacity for DNA amplification reaction chamber these improvements greatly enhance the effectiveness of Xpert Ultra in diagnosing TB [7]. The pooled sensitivity and specificity of Xpert Ultra for TBM diagnosis were 64% and 100%, respectively, and still in comparison Xpert Ultra was more sensitive than Xpert however, both were identical in terms of specificity (100%). This implies that Xpert Ultra is highly effective in diagnosing TBM, and therefore the preferred initial test for TBM [7].
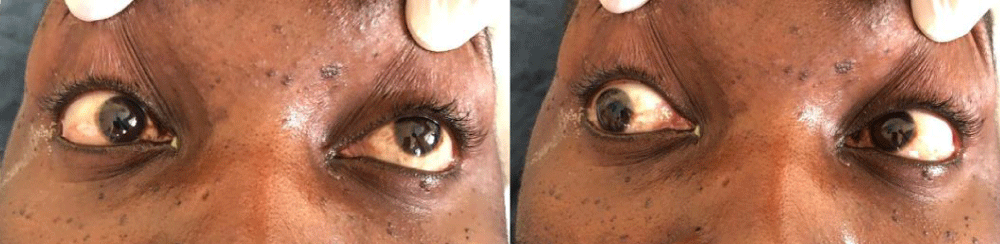
A 33 years old male patient (taxi driver) was admitted via the Orthopedic unit with complaints of progressive lower limb weakness and back pain initially for 3 months. Weakness was more pronounced in the left limb but later involved the right lower limb with associated paresthesia. Symptoms kept on worsening with to inability to walk, stand or support himself and associated urine incontinence and constipation. While on the unit, a diagnosis of spinal mass (meningioma, neurofibroma, nerve sheath tumor) was made however biopsy revealed features of chronic inflammatory process with no granulomas or malignancy but necrotic bone lesions were noted. Postoperatively patient was linked to HIV Clinic following a positive HIV serology. Baseline investigations included, CD4-count of 67cell/µ, urine lipoarabinomannan (LAM) and serum crag were all negative. Serum Toxoplasma IgM/IgG was negative. The patient was started on first-line antiretroviral therapy (ART) with Tenofovir/Lamivudine/Dolutegravir (TDF/3TC/DTG), cotrimoxazole prophylaxis. However, one month later, the patient presented to the emergency room with four days history of a new-onset severe headache, throbbing in nature. Headache was generalized but reportedly more in the frontal area. There was associated confusion, blurring of vision, and multiple episodes of early morning non-projectile vomiting 3 days before admission. He denied a history of convulsions, fever, weight loss, cough, or drenching night sweats. Social history was significant for poor adherence to ART, alcohol intake (5 bottles of beer) with a frequency of about 4 times a week, and an estimated 10 units/week. Examination findings revealed a sick-looking young man, well-nourished with a body mass index (BMI) of 21 kg/m2. The axillary temperature was 37.2 degrees, stage two hypertension of 178/107 mmHg, semiconscious with Glasgow Coma Scale (GCS) of 10/15 (EO = 4, M = 5, V = 2), random blood glucose was 7.6 mmol/l stiff neck, positive Babinski’s but negative Kernig’s sign. Anisocoria of 5 mm on the right and 3 mm on the left. Bilateral 6th cranial nerve palsy and associated right Cranial nerve III palsies as demonstrated in Figure 1. Motor examination was significant for hypotonia in the left upper and lower limbs with normal reflexes. Longitudinal therapeutic, and a non-tender scar extending from T3- T6.
Figure 1: Cranial nerve examination demonstrates Palsy of left abducent nerve palsy and right Cranial nerve III palsy.
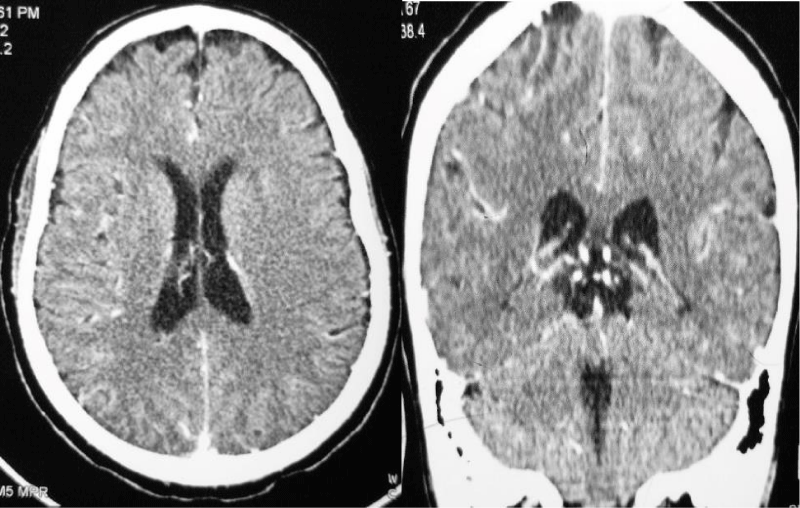
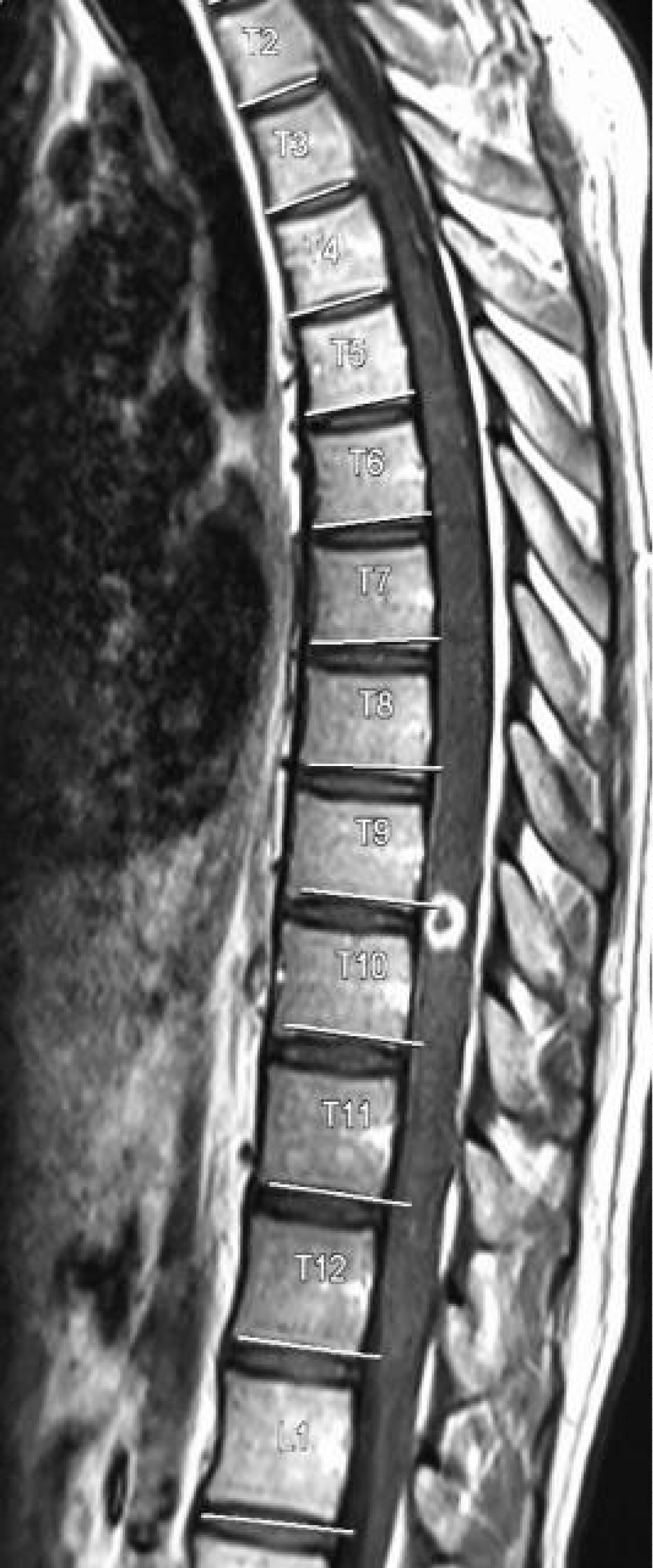
Laboratory investigation showed normal complete blood count, serum electrolytes were remarkable for hyponatremia 129 mmol/L, CD4: 67 cells/µl, CD8: 543 cells/µl, CD4:CD8 Ratio: 0.12, Total CD3: 621 cells/µl. CSF was colorless, with lymphocytic pleocytosis of 80%, proteins were elevated at 182.4 mg/dl, low CSF glucose of 2.20 mmol/l. The ratio of CSF to Serum RBS was 29%. India ink and Ziehl-Neelsen (ZN) for acid-fast bacilli (AFB) were unremarkable for no yeast cells and no AFB respectively. CSF Crag was negative and CSF Gene expert-ultra showed MTB trace detected RIF resistance indeterminate. Contrasted brain CT-Scan, Figure 2 showed diffuse meningeal enhancement with mild hydrocephalus. Spinal Magnetic Resonance imaging demonstrates a ring-enhancing lesion suggestive of spinal tuberculoma and increased signal intensity in thoracic vertebrae T3 to T6 with Gibbus on T2W (Figure 3). The patient was started on anti-tuberculous combination therapy with Isoniazid, Rifampicin, Ethambutol, Pyrazinamide (RHZE), pyridoxine, intravenous dexamethasone, and supportive therapy. He improved dramatically during the 14 days of admission and was discharged in good condition but with neurological sequelae of left lower limb weakness and headache. There were financial constraints with regards to repeating imaging on subsequent reviews.
Figure 2: Contrasted Brain CT scan demonstrates diffuse meningeal enhancement with mild hydrocephalus..
Figure 3: Spinal Magnetic Resonance imaging demonstrates a ring-enhancing lesion suggestive of spinal tuberculoma and increased signal intensity in thoracic vertebrae T3 to T6 and Gibbus on T2W.
The diagnosis of TBM is difficult. Discrimination of cases from those of bacterial meningitis by clinical features alone is often impossible. The delay in diagnosis is usually associated with high mortality and therefore some patients are started empirically on treatment as the diagnosis is being pursued [8].
Typically, patients present with headaches and anorexia, leading to vomiting, photophobia, and fever [3]. In this patient, we see an unusual presentation of lower limb weakness for a protracted course of 4 months and a diagnosis of a spinal mass leading to a biopsy. Microbiologically on CSF analysis, there was lymphocytic pleocytosis of 80%, CSF glucose was low and the ratio of CSF to Serum Glucose was less than 29% highly suggestive of TB if less than 50% in an appropriate clinical setting. This clinical picture is consistent with recommendations from a study by Thwaite, et al. on the diagnosis of adult TBM by use of meningeal signs and low CSF glucose in resource-constrained settings [9]. Another characteristic finding of TBM in this patient was hyponatremia which, affect approximately 50% of patient probably due to cerebral salt wasting and yet more recently, patients who have been diagnosed as having syndrome of inappropriate antidiuretic hormone secretion (SIADH) have been found to have normal levels of antidiuretic hormone [10]. The major source of morbidity from TBM is from vascular complications, particularly cerebral vasculopathy leading to localized ischemic strokes [11]. This patient of concern had grade 2 TBM by modified British Medical Research Council (BMRC). BMRC consists basically of three grades: grade 1, alert and oriented patient without focal deficits; grade 2 disease patients, have a GCS of 10–14 with or without neurological deficits or a GCS of 15 with focal deficits and lastly, patients with grade 3 have a GCS of <10 with or without focal neurological deficits [12].
Direct microscopy of AFB smears is quick and relatively inexpensive. CSF is stained for AFB with the use of the ZN staining technique. However, traditional visualization of CSF to evaluate for AFB by microscopy has been demonstrated to have a poor sensitivity of 10% - 20% [13]. Patients with advanced HIV/AIDS may also be evaluated with the use of LAM testing. LAM is a glycolipid forming component of the MTB cell wall and can be found in multiple body fluids of patients with TB, including CSF, however, this test was negative in this patient of concern. Specifically, in TBM, Xpert MTB/RIF Ultra has demonstrated high sensitivity and specificity, again this result is not affected by centrifugation of CSF. The Tuberculous Meningitis International Research Consortium supports the use of Xpert Ultra, given its superior sensitivity for the diagnosis of TBM as compared to Xpert and culture [14].
The diagnosis of tuberculous meningitis is still challenging in a resource-limited setting and yet delay in diagnosis and treatment greatly increases the mortality. The use of combination clinical assessment, imaging, and a finding of low Glucose in CSF even with the pleiotropic nature of presentation should help in establishing the diagnosis and promptly start treatment. Gene Xpert ultra offers a quick point of test with good sensitivity and specificity to augment clinical decision but if negative should not replace clinical judgment.
- Tuberculosis (TB). https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- WHO. Global Tuberculosis Report. 2020; 148. https://www.who.int/teams/global-tuberculosis-programme/data
- Pormohammad A, Nasiri MJ, McHugh TD, Riahi SM, Bahr NC. A Systematic Review and Meta-analysis of the Diagnostic Accuracy of Nucleic Acid Amplification Tests for Tuberculous Meningitis. J Clin Microbiol. 2019; 57: e01113-1118. PubMed: https://pubmed.ncbi.nlm.nih.gov/30944198/
- van Laarhoven A, Dian S, Ruesen C, Hayati E, Damen MSMA, et al. Clinical Parameters, Routine Inflammatory Markers, and LTA4H Genotype as Predictors of Mortality Among 608 Patients With Tuberculous Meningitis in Indonesia. J Infect Dis. 2017; 215: 1029-1039. PubMed: https://pubmed.ncbi.nlm.nih.gov/28419315/
- Thwaites GE, Bang ND, Dung NH, et al. Dexamethasone for the Treatment of Tuberculous Meningitis in Adolescents and Adults. N Engl J Med. 2004; 351: 1741-1751. PubMed: https://pubmed.ncbi.nlm.nih.gov/15496623/
- Chin JH, Musubire AK, Morgan N, Pellinen J, Grossman S, et al. Xpert MTB/RIF Ultra for Detection of Mycobacterium tuberculosis in Cerebrospinal Fluid. J Clin Microbiol. 2021; 57: e00249-19. PubMed: https://pubmed.ncbi.nlm.nih.gov/30944199/
- Shen Y, Yu G, Zhao W, Lang Y. Efficacy of Xpert MTB/RIF Ultra in diagnosing tuberculosis meningitis: A systematic review and meta-analysis. Medicine (Baltimore). 2021; 100: e26778-e26778. PubMed: https://pubmed.ncbi.nlm.nih.gov/34398057/
- Roth M, Johnson PRA, Rüdiger JJ, King GG, Ge Q, et al. Interaction between glucocorticoids and β2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002; 360: 1293-1299. PubMed: https://pubmed.ncbi.nlm.nih.gov/12414205/
- Thwaites GE, Chau TTH, Stepniewska K, Phu NH, Chuong LV, et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet. 2002; 360: 1287-1292. PubMed: https://pubmed.ncbi.nlm.nih.gov/12414204/
- Foppiano Palacios C, Saleeb PG. Challenges in the diagnosis of tuberculous meningitis. J Clin Tuberc other Mycobact Dis. 2020; 20: 100164. PubMed: https://pubmed.ncbi.nlm.nih.gov/32462082/
- Belorgey L, Lalani I, Zakaria A. Ischemic Stroke in the Setting of Tuberculous Meningitis. J Neuroimaging. 2006; 16: 364-366. PubMed: https://pubmed.ncbi.nlm.nih.gov/17032389/
- Thwaites GE, Hien TT. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005; 4: 160-170. PubMed: https://pubmed.ncbi.nlm.nih.gov/15721826/
- Bahr NC, Boulware DR. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med. 2014; 8: 1085-1103. PubMed: https://pubmed.ncbi.nlm.nih.gov/25402579/
- Seddon JA, Tugume L, Solomons R, Prasad K, Bahr NC, et al. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome open Res. 2019; 4: 167. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029758/