More Information
Submitted: August 09, 2021 | Approved: August 20, 2021 | Published: August 23, 2021
How to cite this article: Coculescu BI, Coculescu EC. Evaluation of microbial contamination in a surgical department of a Romanian military emergency hospital - A case study. J Clin Intensive Care Med. 2021; 6: 026-028.
DOI: 10.29328/journal.jcicm.1001037
Copyright License: © 2021 Coculescu BI, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Microbiological air contamination; Sanitary analysis; Sedimentation method; E. coli
Evaluation of microbial contamination in a surgical department of a Romanian military emergency hospital - A case study
Bogdan-Ioan Coculescu1,2* and Elena Claudia Coculescu3
1Cantacuzino National Medico-Military Institute for Research and Development, Bucharest, Romania
2Titu Maiorescu University, Faculty of Medicine, Bucharest, Romania
3Carol Davila University of Medicine and Pharmacy, Faculty of Dental Medicine, Bucharest, Romania
*Address for Correspondence: Bogdan-Ioan Coculescu, Cantacuzino National Medico-Military Institute for Research and Development, 103 Splaiul Independenței, 050096, Bucharest, Romania, Email: [email protected]
Assessment of the microbial load of the operating environment during daily pre-, intra-, and post-operative procedures in a surgical department of a military emergency hospital in Bucharest showed the bacterial contamination of intra-operative air by increasing the number of bacteria above the allowed maximum level and the detection of a strain of Escherichia coli (E. coli).
The present study aimed at assessing the risk of exposure of patients and health professionals through the quantitative and qualitative detection of possibly pathogenic aerobic micro-organisms which may accidentally contaminate the existing microclimate during intra-operative procedures [1-6]. Research into the microbial load of the operative environment during daily pre-, intra-, and post-operative procedures in a surgical department of a military emergency hospital in Bucharest were carried out in the specialized laboratory for microbiological diagnosis only. In this reason, in October 2014, in an 8-hour period, the microbial load in an operating block was determined and monitored by two specific working methods during pre- and post-operative procedures, respectively, in a surgical department of a military hospital in Bucharest.
Quantitative analysis
a) The microbiological surface control aimed at assessing the following areas: the operating table, the operating tray, the “Y” piece of the respiratory tube connected to the peri-operative monitoring equipment (Dräger), intravenous poles and monitor keyboard. The method of wiping the surface with a sterile cotton swabs (TIS), moistened with 1 ml saline before use, was used. Each area for the collection of microbiological samples corresponded to a square with a 10 cm side (delimited by labelling). Subsequently the swab was placed in a test tube with 9 ml sterile saline.
The determination of the total plate count (TPC) was carried out by the plate counting method and consisted of performing decimal dilutions (1:10) of each raw suspension followed by the incorporation of 1 ml of these samples (crude suspension and decimal dilution) into 2% melted and cooled agar at 45 to 50°C, poured into Petri dishes, followed by incubation at 37°C and a reading of the number of colonies developed on the two plates of each sample after 48 hours. The results were expressed as micro-organisms/cm2 of test surface.
For the detection of Escherichia coli (E. coli), 2 ml of the raw dilution was placed in lactose broth with a Durham fermentation tube and in the case of tubes which fermented the lactose by producing gas, they were transferred to the Levine medium. Biochemical identification has been used in the multi-test media, TIS (triple sugar iron), MIU (motility, indole, urea), MILF (mobility, indole, lysinedecarboxylase, phenylalaninedeaminase), and Simmons citrate, respectively. Confirmation of Escherichia species was performed by: examination of morphology and mobility, study of growth at 37 °C (microaerob) and 44.5°C (aerobic).
For the isolation of micro-organisms of the genus Proteus, 1 ml of raw dilution in the condensate of an agar slant tube incubated in an upright position was grown and the phenomenon of “climbing” was observed after 24 hours. (development of a pure culture in “overlapping waves” to the top of the culture medium), and for the determination of Staphylococcus aureus, 2-3 ml of the raw suspension was mixed in equal parts with the liquid (Chapman) medium and after a 24-hour incubation at 37 °C, it was transferred to a solid Chapman medium and 10% blood agar.
b) In parallel, the bacterial concentration in the air - aeromicroflora (AMF) - was determined by the Koch sedimentation method. The culture medium used was: nutritive agar (mesophilic bacteria), agar with blood 5% - 10% (hemolytic bacteria) and Sabouraud agar (fungi). A number of 3 groups of Petri dishes (d = 9 cm) were used, each group comprising a plate with 2% nutrient agar (Oxoid) and pH = 7.4-7.6, and a plate with 10% blood agar, both media being freshly prepared and dried to ensure that there is no condensation liquid on the surface. One group of plates was exposed in the middle of the research room, on the operating table, the second group was exposed in one corner of the operating room and the third group at the window sill. Exposure was made by lifting the lids of the Petri dishes and placing them, opening downwards, next to the medium plates. The exposure time was 10 minutes from when the lids were lifted. After exposure, the Petri dishes were put in thermostat at 37 °C for 24 hours for blood agar and a further 24 hours at room temperature for plain agar. At the same time, a group of medium plates, prepared from the same batch, but not exposed (control) were incubated. In the case of determination of the microbial flora in the air, the following criteria were observed as bacteriological indicators: number of bacteria/m3 of air, and number of hemolytic bacteria (pathogen staphylococcus, β-hemolytic streptococcus/m3 of air). The reference to m3 of air was made by applying the Omelianski formula.
Qualitative analysis
The identification of pathogenic bacteria was performed based on the culture, morphotinctorial and biochemical properties characteristics.
From the point of view of microbial load, the results of the surface control are given in Table 1.
| Table 1: Microbiological load of the surfaces in the operating block. | |||||||
| Total plate count/cm2 | S. aureus, E. coli, Proteus | ||||||
| No. | Surface tested |
Pre-operative* | Post-operative | Pre- operative |
Pre-operative | Post-operative | Pre- operative |
| 1. | Operating table | 2 | 5 | 1 | 0 | 1 | 0 |
| 2. | Operating tray | 1 | 4 | 2 | 0 | 0 | 0 |
| 3. | “Y” piece | 2 | 4 | 2 | 0 | 0 | 0 |
| 4. | Intravenous pole | 2 | 7 | 1 | 0 | 0 | 0 |
| 5. | Monitor keyboard | 1 | 3 | 2 | 0 | 0 | 0 |
| *pre-operative = 10-15 min. after cleaning and disinfection. *post-operative = at the end of the working day. |
|||||||
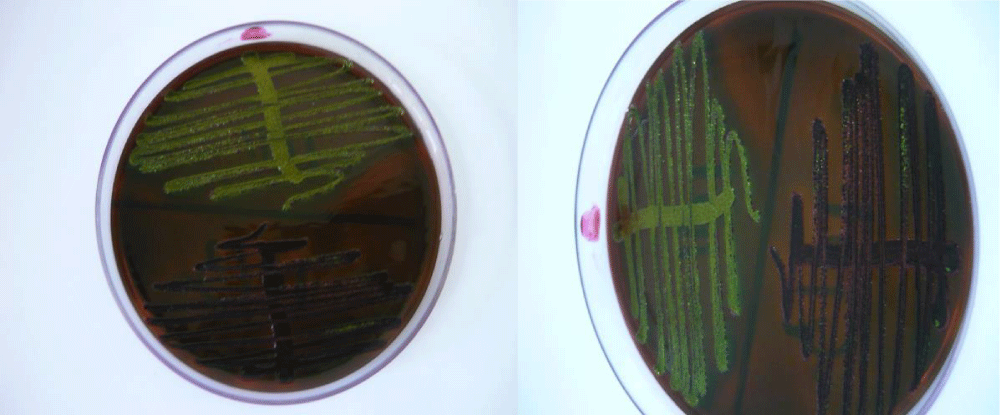
The classical microbiological analysis confirmed the existence of E. coli on the operating table (Figure 1).
Figure 1: Strain of E. coli isolated from the operating table in the Levine Eosin Methylene Blue Agar medium (EMBA) - characteristic, lactose-positive (dark purple) colonies with metallic sheen.
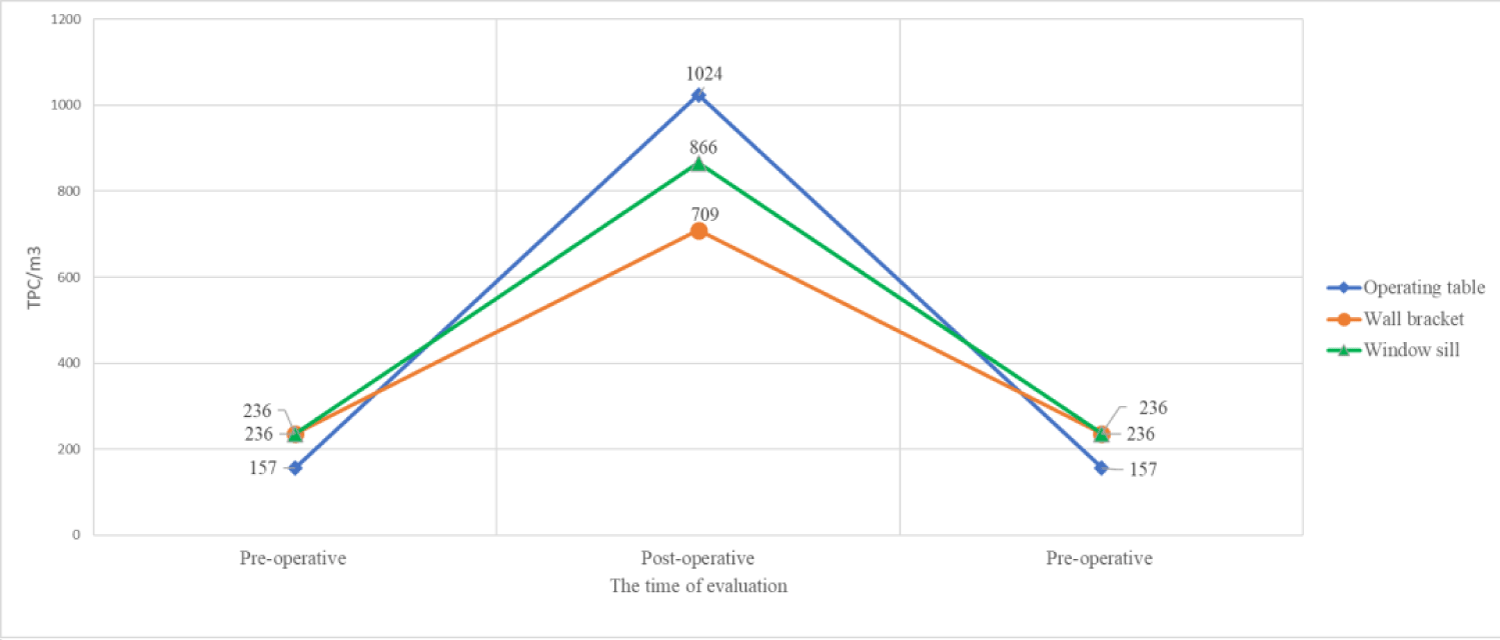
Figure 2: Degree of microbial contamination of air within 8 hours in the operating block of a surgical department.
For air control for microbial load (AMF) the results obtained in the operating block are given in Table 2.
| Table 2: Microbiological load of the air in the operating block (exposure time = 10 min). | ||||||
| Name of controlled area | Pre-operative (10-15 min. after cleaning and disinfection) | Post-operative (at the end of the working day) | ||||
| TPC*/ m3 | S. pathogen/ 1 plate |
Str. β-hemol./ 1 plate |
TPC/m3 | S. pathogen /1 plate | Str. β-hemol./ 1 plate |
|
| Operating table | 157** | 0 | 0 | 1024 | 0 | 0 |
| Wall bracket | 236 | 0 | 0 | 709 | 0 | 0 |
| Window sill | 236 | 0 | 0 | 866 | 0 | 0 |
| *TPC= total plate count. **according to the Omelianski formula N x 10000 = no. of bacteria/m3 air S x K (Where N = colony count on the surface of the Petri dish, S = the surface of the medium in cm2 and K = 2). |
||||||
Bacterial load in the air in the monitored operating block within 8 hours is shown in Figure 2.
The control plates did not show colonies.
Interpretation of the results was carried out by comparing them with the bacteriological indicators established in medical legislation.
The surfaces tested showed a microbial load at 37 °C (TPC) of 1-2 × 104 CFU/m2 and no pathogenic micro-organisms (S. aureus, E. coli or Proteus) were identified, the results being within the maximum allowed limits. (CFU mean colony forming unit).
Post-operative, the same areas showed a degree of contamination range of 3-7 x 104 CFU/m2, which were not within the maximum admissibility limits. In addition, one of the surfaces tested was also contaminated with E. coli.
Subsequently, at the 15-minute control after cleaning and disinfection of the surfaces concerned, the microbial load values returned to the acceptability limits of microbiological contamination recommended by the rules in force.
The total plate count (TPC) that developed at 37 ºC/m3 of air collected from different areas of the surgical room showed a significant increase at the end of the working day from 157 - 236 to 709 - 1024 bacteria/m3 of air (i.e. > 400% of the starting values), above the maximum admissibility limits. However, no bacteria were identified of the Staphylococcus aureus and Streptoccocus β-hemolytic strains.
Intra-operative transmission of a pathogenic bacterial strain by air and consequently contamination of the operating room was confirmed by this study. Thus, the risk of healthcare-associated infections is also favoured by healthy carriers of pathogens present in the general population.
The results of the tests carried out in this study by biological monitoring of the microclimate during the day showed an increase in hygienic-sanitary values beyond normal limits and the identification of one pathogenic bacteria, E. coli. In order to avoid such situations, which may lead to contamination in operating rooms of both patients and surgical teams, regular checks should be carried out in the future by taking hygienic and sanitary swab samples (including of the surgical instruments) and of aeromicroflora throughout the day and analysing them in the microbiology laboratory.
The low frequency of hygienic-sanitary monitoring in the operating rooms is primarily a management (organizational) issue and not a financial one, because both types of hygienic-sanitary tests (TIS and AMF) are available at affordable prices/costs.
This study showed that patients have the potential to transmit potentially pathogenic microorganisms with intestinal localization to the surgical staff.
In the post-operative environment, there has been a significant increase (about 200% - 300% above the allowed limits) of the total number of mesophilic bacteria (TPC), which heavily contaminated the air and the test surfaces, respectively, in the aseptic operating room. The operating table showed the highest aerobic microbial contamination and additionally a pathogenic micro-organism (E. coli) was isolated on it.
The presence of E. coli is a risk factor for the appearance of healthcare-associated infections. It would have been useful to compare the microflora of the wound in case of the appearance of possible suppuration after aseptic surgery with the microflora on the surfaces and the air in the operating room, but this was not possible because the patients in the department were discharged after 24 hours.
A correlation has been established between the microbial load of surfaces and that of the air, showing a risk of contamination directly proportional to the TPC and the presence of pathogenic micro-organisms. In this context, it is recommended to follow the measures regarding hand hygiene, cleaning and disinfection with medium-high level disinfectants being carried out not only at the end of the working day but also between surgical interventions.
- Tanner WD, Leecaster MK, Zhang Y, Stratford KM, Mayer J, et al. Environmental Contamination of Contact Precaution and Non-Contact Precaution Patient Rooms in Six Acute Care Facilities. Clin Infect Dis. 2021; 72: S8-S16. PubMed: https://pubmed.ncbi.nlm.nih.gov/33512527/
- Huang J, Cui C, Zhou S, Chen M, Wu H, et al. Impact of multicenter unified enhanced environmental cleaning and disinfection measures on nosocomial infections among patients in intensive care units. J Int Med Res. 2020; 48: 300060520949766. PubMed: https://pubmed.ncbi.nlm.nih.gov/32820692/
- Squeri R, Genovese C, Trimarchi G, Antonuccio GM, Alessi V, et al. Nine years of microbiological air monitoring in the operating theatres of a university hospital in Southern Italy. Ann Ig. 2019; 31: 1-12. PubMed: https://pubmed.ncbi.nlm.nih.gov/30994159/
- Farhadloo R, Goodarzi Far J, Azadeh MR, Shams S, Parvaresh-Masoud M. Evaluation of Bacterial Contamination on Prehospital Ambulances Before and After Disinfection. Prehosp Disaster Med. 2018; 33: 602-606. PubMed: https://pubmed.ncbi.nlm.nih.gov/30376910/
- West GF, Resendiz M, Lustik MB, Nahid MA. Bacterial Contamination of Military and Civilian Uniforms in an Emergency Department. J Emerg Nurs. 2019; 45: 169-177. PubMed: https://pubmed.ncbi.nlm.nih.gov/30573161/
- Order by Ministry of Health no. 1101/2016 regarding the approval of the surveillance, prevention and limitation of healthcare associated infections in hospitals [in Romanian]. 2016.